Refractory vascular anomalies (tumors/malformations)
■Principal investigator
Michio Koseki / Department of Pediatrics, Gifu University Hospital
Disease Description
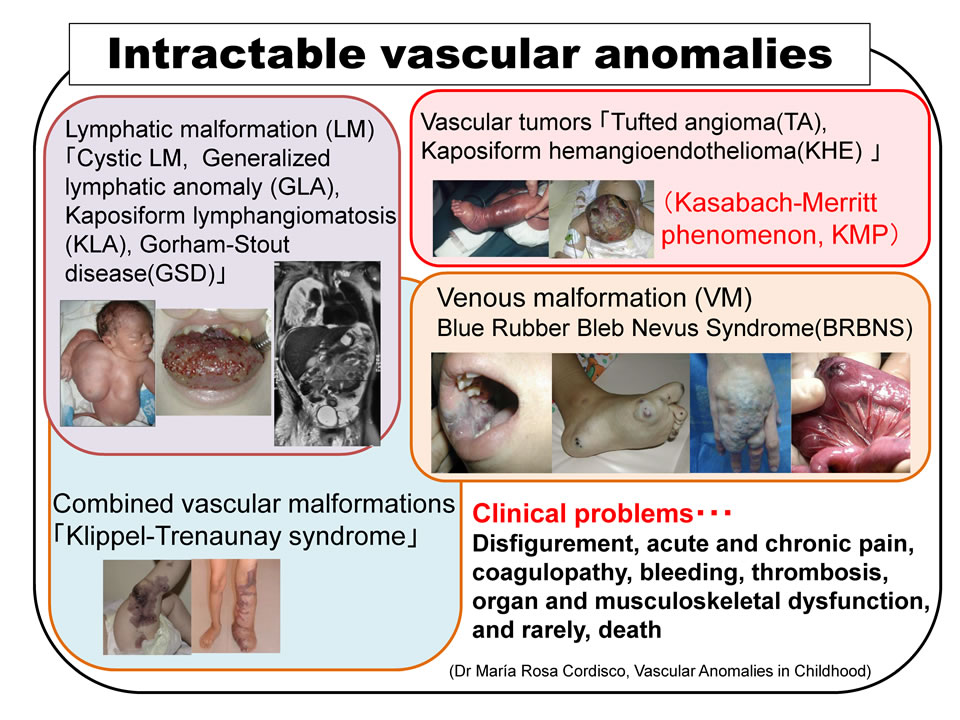
Vascular tumors and malformations (collectively, “vascular anomalies”) refer to abnormal formations of blood and lymph vessels—respectively called “hemangiomas” and “lymphangiomas” in clinical settings—which typically develop in childhood. “Refractory vascular anomalies” refer to a severe subset of cases involving extensive, unresectable lesions in the face or limbs causing cosmetic or functional impairment, infiltration of the neck or chest causing fatal respiratory distress, or severe hemorrhagic tendencies, such as in Kasabach–Merritt phenomenon (KMP). Representative examples include Kaposiform hemangioendothelioma and tufted angioma, which cause KMP, as well as lymphangioma (recently called “lymphatic malformation”), lymphangiomatosis, Gorham’s disease, venous malformation, blue rubber bleb nevus syndrome, mixed vascular malformation, and Klippel–Trènaunay–Weber syndrome.
Sirolimus: Expected Effects and Efficacy
Development is underway of novel therapeutic drugs targeting the PI3K/AKT/mTOR pathway, the mutations in which have recently been implicated in the etiology and pathology of vascular anomalies. Sirolimus has attracted particular attention as a novel therapeutic drug for vascular anomalies, reported to demonstrate efficacy in 47/57 cases (82.5%) in an American clinical trial started in 2009. The subsequent cascade of positive research reports has fueled hopes in its therapeutic efficacy. Sirolimus can reduce lesion size and improves symptoms in many types of vascular anomaly.
Research Progress
Seeking expanded indications for sirolimus, our research group partnered with NobelPharma Co. Ltd. in 2015 and was awarded AMED funding in 2016 under the project, “Research to Establish Sirolimus as a Therapy for Refractory Lymphatic Anomalies” (AMED Project Promoting Clinical Research and Trials). In 2017, we performed a multicenter phase 3 physician-led trial to assess the efficacy and safety of NPC-12T (sirolimus) in the treatment of refractory lymphatic diseases. We are also conducting a multicenter open-label uncontrolled study to assess the efficacy and safety of sirolimus in the treatment of intractable vascular anomalies (Specified Clinical Trial, jRCTs031180290), as well as a multicenter phase 3 physician-led clinical trial assessing the efficacy and safety of NPC-12T (granules/tablet) in the treatment of refractory hemangioma/vascular malformations. If regulatory approval is granted based on these trials, it will mark the world’s first authorization of sirolimus to treat vascular anomalies.
In the course of developing sirolimus-based treatments, our research group is working on population-level pharmacokinetic analysis using valuable data—as well as preclinical research using model mice, determining its pharmacological action, conducting genetic analyses, and searching for biomarkers—with the goal of establishing safe and effective dosage forms, especially for children. Our goals are conducting drug development research on sirolimus—an innovative targeted molecular therapy for refractory hemangioma and vascular malformations—based on globally pioneering, novel clinical research and trials and conducting research that will bring about new “genomic medicine for vascular anomalies” involving specific gene targets.
Related Links
■Lymphatic Disease Information Station
(Providing patients with information about lymphatic diseases, such as lymphangioma, lymphangiomatosis, and Gorham’s disease)
http://www.lymphangioma.net/
■The International Society for the Study of Vascular Anomalies (ISSVA) - Homepage
https://www.issva.org/